 |
Enrichment
of Uranium
U235 is available in nature only to a limited
extent of 0.7% of natural Uranium. To acquire weapons grade U235,
Uranium must be enriched to 90%. In order to fuel a bomb, the
U-235 must be separated from the more abundant U-238 isotope in
a process called enrichment. But because these two uranium isotopes
are almost identical chemically, they cannot be readily separated
by using a simple chemical reaction. They must be separated by
exploiting the slight difference in their weights.
There are several methods developed for
this: two are commercial - gaseous diffusion process and the centrifuge
process.
USNRC
Uranium Enrichment
Uranium Information
Center

|
 |
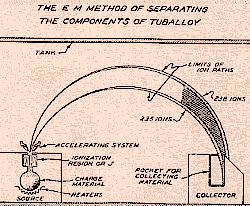
Electro Magnetic Separation
This is a schematic of the method developed
by E.O.Lawrence. Passing ions of Uranium threw a magnetic
field causes the U235 ions to take a different path than the
U238. Collectors at the other end of the semi-circle collect
the separated U235.
|
 |
Fission of U-235
The minimum amount for a critical mass
for U-235 is 52Kg, for Pu-239 it is 10Kg.
Neutron fired into a U-235 atom creating
a U-236 atom.
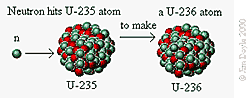
The resulting U-236 is unstable and will
rapidly decay.
|
 |
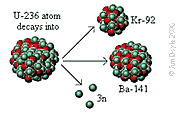
Decay
The decay of U-236 into an atom of Ba-141
(barium), an atom of Kr-92 (krypton) and three neutrons releases
a significant amount of energy.
|
 |
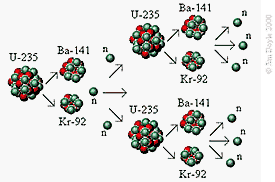
Resulting Chain Reaction
The three available neutrons collide with
other U-235 atoms causing them to fission producing more free
neutrons. This process continues creating a chain reaction in
the (critical) mass of the U-235 Isotope. The mass can fission
quickly as the number of split atoms grows exponentially.
|
 |
Energy Released
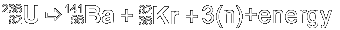
The left side of the equation has a mass
of 236.045563 and the right side totals to 233.849298. The difference
between the masses (2.196265) must, by Einstein's equivalence
principle, appear as energy released in the reaction. This energy
is represented as intense heat. 200MeV with each fissioned molecule.
1 MeV (million electron volts) = 1.609 x 10 -13 joules
Though the mass is small, the energy is
calculated by multiplying the speed of light squared, then multiplied
by the number of atoms that fission during the process!
More on Nuclear Fission
USAF
Academy online lesson in atomic bomb physics
|
 |


Nuclear
Weapons Frequently Asked Questions
Nuclear
Weapons
|
 |
Little Boy

Considered a gun-type bomb. A disc of uranium 235 is shaped with
a center section missing. The center bullet, is place down the
barrel from the larger ring mass. A conventional explosive is
used to propel the center section into the larger ring section.
The two sections then come together forming a critical mass and
starting the reaction. The barometric sensor detects the height
from the surface of the Earth by measuring air pressure. This
allowed the bomb to be exploded above the ground reulting in more
damage.

|
 |
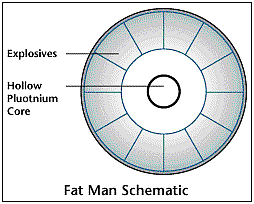
Fat Man

A Beryllium/Polonium mixture, which is radioactive elements that
release neutrons, is placed in the center of a sphere. The sphere
is made up of equally spaced and shaped plutonium sections. The
sphere looked a lot like a soccer ball. When the bomb was detonated,
the sphere implodes, or collapses inward, causing all the plutonium
to fuse together thus reaching supercritical mass, and starting
the chain reaction. The initial explosion, which caused the implosion,
was made by conventional explosive placed evenly on the outside
of the sphere.
|
  |
|


![]()